A) trigonal bipyramidal
B) octahedral
C) square pyramidal
D) square planar
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which has the smallest dipole-dipole forces?
A) CH3F
B) HCl
C) N2
D) CO
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would be expected to have sp2 hybridization on atom A? 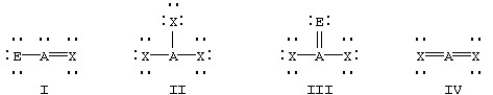
A) II
B) I and III
C) I,II,and III
D) I and IV
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the bond angles in the following molecular model of BBr3? 
A) less than 109.5°
B) 109.5°
C) less than 120° but greater than 109.5°
D) 120°
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound below could have a zero dipole moment?
A) CCl2F2 (tetrahedral)
B) CuCl2F2 (tetrahedral)
C) PtCl2F2 (square planar)
D) SCl2F2 (see-saw)
F) All of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
Helium can be liquefied when He atoms are attracted to one another by intermolecular ________ forces.
Correct Answer

verified
dispersion...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
The following ball-and-stick molecular model is a representation of the amino acid alanine (unshaded spheres = H) .Only the connections between atoms are shown;multiple bonds and nonbonded electrons are not indicated. 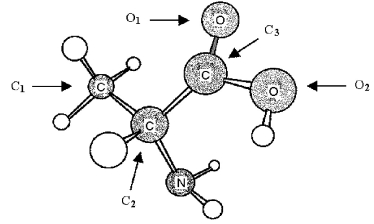 -In order to complete an electron-dot structure of alanine,the oxygen atom labeled O2 needs
-In order to complete an electron-dot structure of alanine,the oxygen atom labeled O2 needs
A) 1 additional bond and 1 nonbonded pair of electrons.
B) 1 additional bond and 2 nonbonded pairs of electrons.
C) 1 nonbonded pair of electrons.
D) 2 nonbonded pairs of electrons.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule has a central atom that uses the set of hybrid orbitals shown below to form bonds with the non-central atoms? 
A) H2Se
B) CS2
C) NO2-
D) ICl2-
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following should have the largest dipole moment?
A) F2(g)
B) SO2(g)
C) RbBr(g)
D) CH2I2(g)
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following MO diagram is appropriate for Li2 and Be2.Based on this diagram, 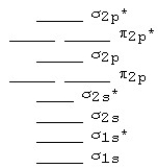
A) both are stable and diamagnetic.
B) Li2 is stable and diamagnetic,but Be2 is unstable.
C) Be2 is stable and diamagnetic,but Li2 is unstable.
D) Be2 is stable and paramagnetic,but Li2 is unstable.
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of SbCl3?
A) T-shaped
B) tetrahedral
C) trigonal planar
D) trigonal pyramidal
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which drawing represents the molecular orbital containing the highest energy electrons in the H2 molecule in its ground state?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the geometry around the central atom in the following molecular model of NO2-? 
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Shown below is a model of CBr4 having an orientation in which one or more atoms are hidden from view.What is the indicated bond angle? 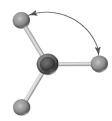
A) less than 109.5°
B) 109.5°
C) less than 120° but greater than 109.5°
D) 120°
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the bond angles in the following molecular model of XeF4? 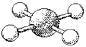
A) some less than 90° and some less than 120° but greater than 90°
B) 90°
C) some less than 90° and some less than 180° but greater than 90°
D) 90° and 180°
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of SeF5-?
A) octahedral
B) seesaw
C) square pyramidal
D) trigonal bipyramidal
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the set of hybrid orbitals shown below. 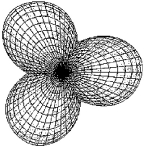
A) sp
B) sp2
C) sp3
D) sp4
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which drawing represents the molecular orbital containing the highest energy electrons in the O22- molecular ion in its the ground state?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
The polarity of CCl4 is ________.
Correct Answer

verified
Correct Answer
verified
Short Answer
Are the π-bonds in CH3CO2- delocalized or localized?
Correct Answer

verified
Correct Answer
verified
Showing 121 - 140 of 205
Related Exams