A) to the right
B) to the left
C) zero
E) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these square planar complex ions can have cis-trans isomers?
A) [Pt(NH3) 4]2+
B) [Ni(NH3) 4]2+
C) [Pt(NH3) 2Cl2]
D) [Pt(NH3) Cl3]-
E) [Ni(NH3) 3Cl]+
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The systematic name of the coordination compound K2[Co(H2O)2I4] is potassium diaquotetraiodocobaltate(II).
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following ions could exist in only the high-spin state in an octahedral complex?
A) Cr2+
B) Mn4+
C) Fe3+
D) Co3+
E) Ni2+
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 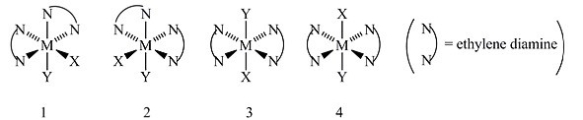 Which, if any, of the following pairs are optical isomers?
Which, if any, of the following pairs are optical isomers?
A) Structures 1 and 2
B) Structures 1 and 3
C) Structures 1 and 4
D) Structures 3 and 4
E) Structures 1, 2, 3, and 4
G) All of the above
Correct Answer

verified
Correct Answer
verified
True/False
In complexes of transition metals, the maximum coordination number of the metal is equal to its number of d electrons.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a bidentate ligand?
A) cyanide ion
B) nitrite ion
C) ammonia
D) thiocyanate ion
E) ethylenediamine
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many unpaired electrons are there in the complex ion [Mn(CN) 6]3-?
A) 0
B) 1
C) 2
D) 3
E) 5
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following octahedral complexes should have the largest crystal field splitting energy, Δ?
A) [Cr(H2O) 6]3+
B) [Cr(SCN) 6]3
C) [Cr(NH3) 6]3+
D) [Cr(CN) 6]3-
E) [Cr(en) 3]3+ (en = ethylenediamine)
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A bidentate ligand always
A) forms bonds to two metal ions.
B) has a charge of 2+ or 2-.
C) forms complex ions with a charge of 2+ or 2-.
D) has two donor atoms.
E) has medical uses.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
In a reaction between a metal ion and a group of anions or a polar molecule, the metal ion acts as a ________ ________.
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the oxidation number of Fe in [Fe(CN)6]4-?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What name is given to the species that has the ability to hold the metal atom, in the complex ion, like a claw?
A) Claw complex
B) Grasp agent
C) Chelating agent
D) Clutch ligand
E) Clasp a ligand
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many 4d electrons does a ground-state Ag+ ion have?
A) 8
B) 1
C) 10
D) 2
E) 9
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following energy-level diagram could correspond to which coordination compound? 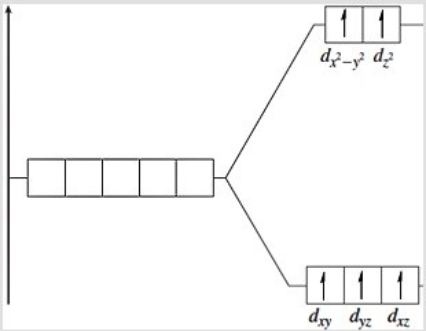
A) hexabromomanganate(II)
B) hexacyanoferrate(II)
C) hexacyanoferrate(III)
D) hexabromomanganate(IV)
E) hexachlorochromate(II)
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name that refers to the number of donor atoms surrounding the central metal atom in a complex ion?
A) Donor number
B) Coordination number
C) Metal number
D) Metal-donor ratio
E) Acceptor number
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the coordination compound [Cr(NH3) 2(en) Cl2]Br2, the coordination number (C.N.) and oxidation number (O.N.) of the metal atom, respectively, are
A) C.N. = 6; O.N. = +4.
B) C.N. = 6; O.N. = +3.
C) C.N. = 5; O.N. = +2.
D) C.N. = 5; O.N. = +4.
E) C.N. = 4; O.N. = +3.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the complex ion [Co(en) 2Br2]+, what is the oxidation number of Co?
A) +1
B) +2
C) +3
D) -2
E) -1
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following structures (1 and 2 are octahedral; 3 and 4 are square planar) . 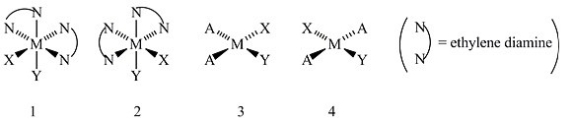 Which one of the following statements about the above structures is correct?
Which one of the following statements about the above structures is correct?
A) Structures 1 and 2 are superimposable.
B) Structures 1 and 2 are geometric isomers.
C) Structures 3 and 4 are structural isomers.
D) Structures 3 and 4 are optical isomers.
E) Structures 3 and 4 are geometric isomers.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
The atom in a ligand that is bound directly to the metal atom is known as the ________ ________.
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 132
Related Exams