A) H2O
B) BCl3
C) CH4
D) CO2
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formal charge of the carbon in carbon dioxide (CO2) when drawn with two double bonds?
A) +1
B) 0
C) -1
D) -2
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When forming molecular orbitals from atomic orbitals,what is the order of increasing C-H bond strength for the following set? 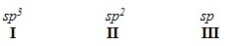
A) II < I < III
B) III < I < II
C) III < II < I
D) I < II < III
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atomic orbitals overlap to form the C-H s bonding molecular orbitals of ethylene,H2C=CH2?
A) C2p + H1s
B) Csp + H1s
C) Csp3 + H1s
D) Csp2 + H1s
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about electronegativity and the periodic table is true?
A) Electronegativity decreases across a row of the periodic table.
B) Electronegativity increases down a column of the periodic table.
C) Electronegativity increases across a row of the periodic table.
D) Electronegativity does not change down a column of the periodic table.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound contains the most polar bond? 
A) I
B) II
C) III
D) IV
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following Lewis structures is correct? 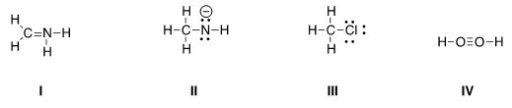
A) I,II
B) I,III
C) II,III
D) III,IV
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the appropriate conversion of (CH3) 2CHOCH2CH2CH2OH to a skeletal structure? 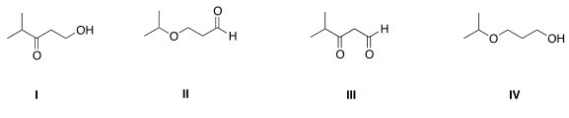
A) I
B) II
C) III
D) IV
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are around phosphorus in phosphoric acid (H3PO4) ?
A) 6
B) 8
C) 10
D) 12
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has non-polar covalent bonds?
A) CO2
B) N2
C) CCl4
D) HF
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the appropriate conversion of the condensed structure,CH3COCH3,to a Lewis structure? 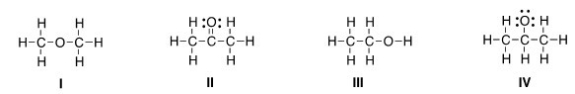
A) I
B) II
C) III
D) IV
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the geometry around the indicated atom in each species. 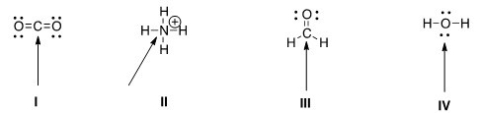
A) I = Linear; II = tetrahedral; III = trigonal planar; IV = tetrahedral
B) I = Linear; II = tetrahedral; III = trigonal planar; IV = linear
C) I = Trigonal planar; II = linear; III = tetrahedral; IV = trigonal planar
D) I = Tetrahedral; II = trigonal planar; III = linear; IV = tetrahedral
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would you expect to have ionic bonds?
A) CO
B) FBr
C) NF3
D) NaCl
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many constitutional isomers are there for a molecule having the molecular formula C2H6O?
A) 1
B) 2
C) 3
D) 4
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about resonance structures is true?
A) Resonance structures have the same placement of electrons but different arrangement of atoms.
B) Resonance structures have the same placement of atoms but different arrangement of electrons.
C) Resonance structures have the same placement of atoms and the same arrangement of electrons.
D) Resonance structures have different placement of atoms and different arrangement of electrons.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the nitrogen atom in the ammonium cation,NH4+?
A) sp3
B) sp2
C) sp
D) p
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following atoms in order of increasing electronegativity,putting the least electronegative first. 
A) I < II < III < IV
B) I < IV < II < III
C) III < II < IV < I
D) I < II < IV < III
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 77 of 77
Related Exams